Gaseous State | Introduction#
Gaseous State | Introduction#
In the chapter of gaseous state, we will mainly focus on three things: (a) Ideal Gas Equation (b) Kinetic Theory of Gases and (c) Real Gases.
Volume of a container#
-
The maximum amount of quantity contained by the container is known as its volume.
-
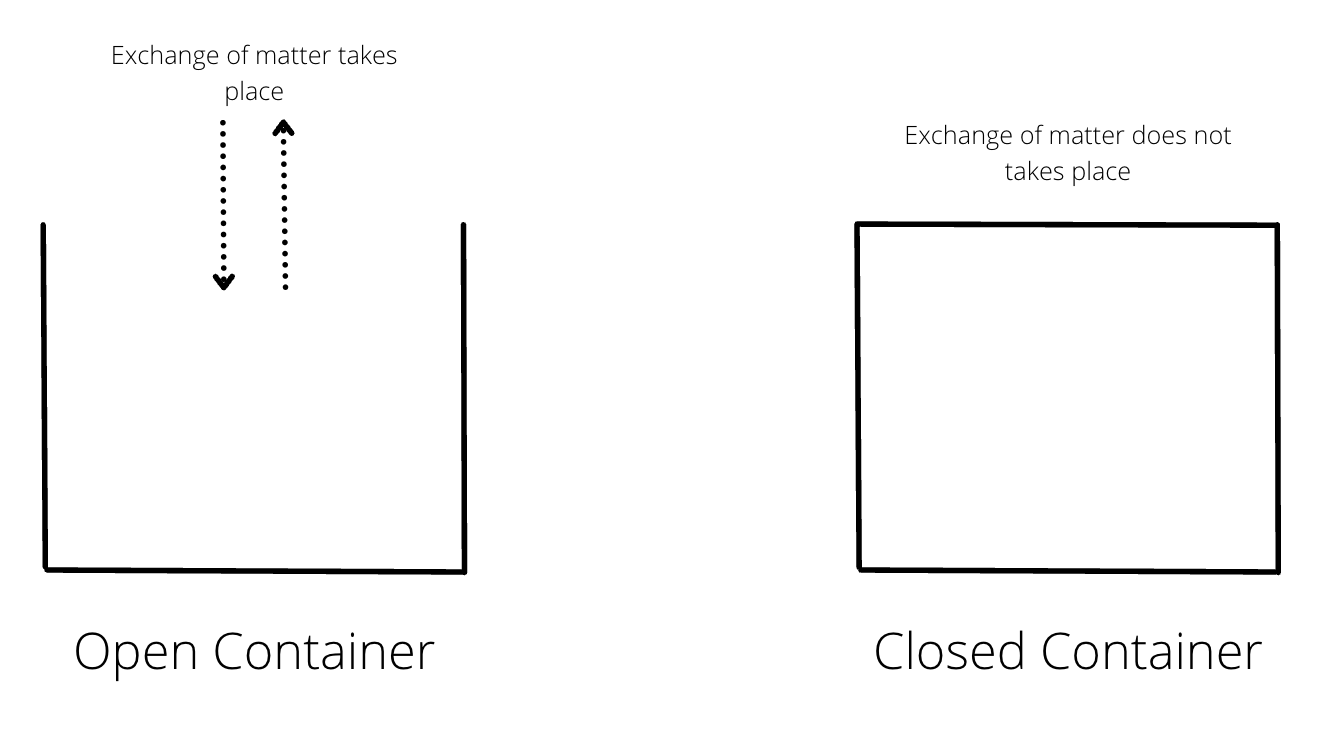
There are two types of containers - open container and closed container.
-
Open containers allow exchange of both matter and energy while closed containers do not allow exchange of matter but can allow exchange of energy through walls.
-
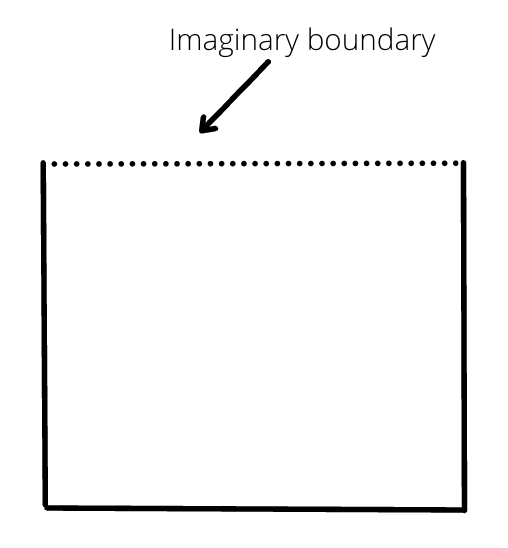
While calculating volume of the open container, we consider an imaginary boundary at the open end.
- There are several units of volume: cubic centimetre(cm3), cubic metre(m3), millilitres(mL), litres(L). The standard unit of volume is litre(L).
Pressure#
- Force (F) applied per unit area (A) is known as pressure (P). Mathematically, it can be stated as:
-
The SI unit of pressure is Newton per square metre (N/m2) or Pascal (Pa).
-
There are other units of pressure such as atm, bar, torr etc.
Remember
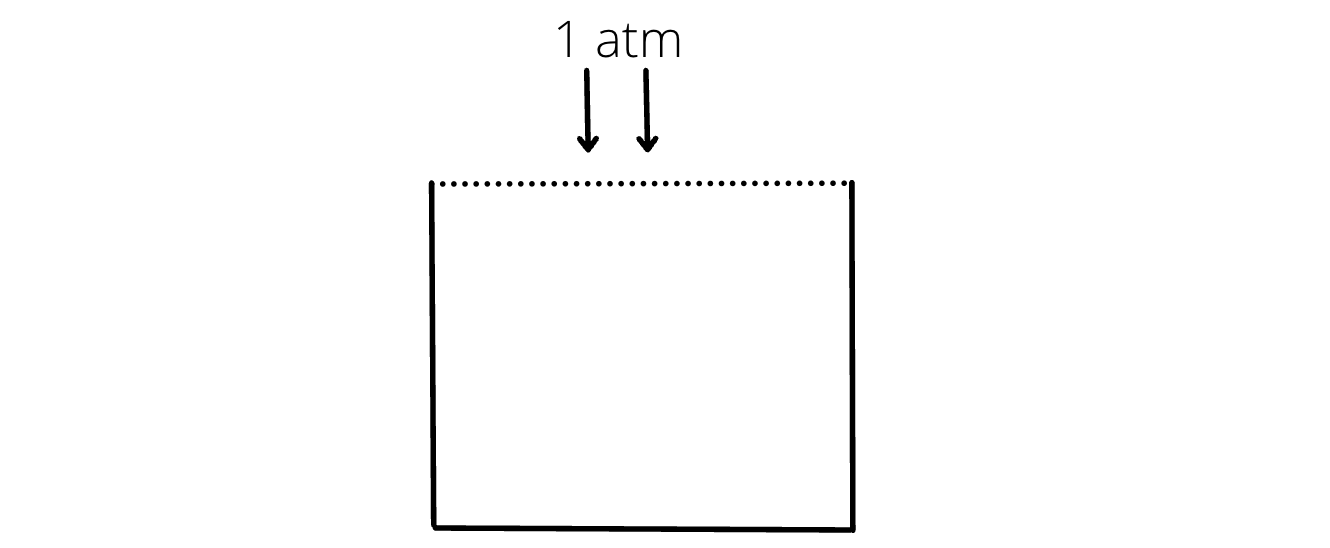
- At the open end of a container under normal conditions, pressure is always 1 atm.
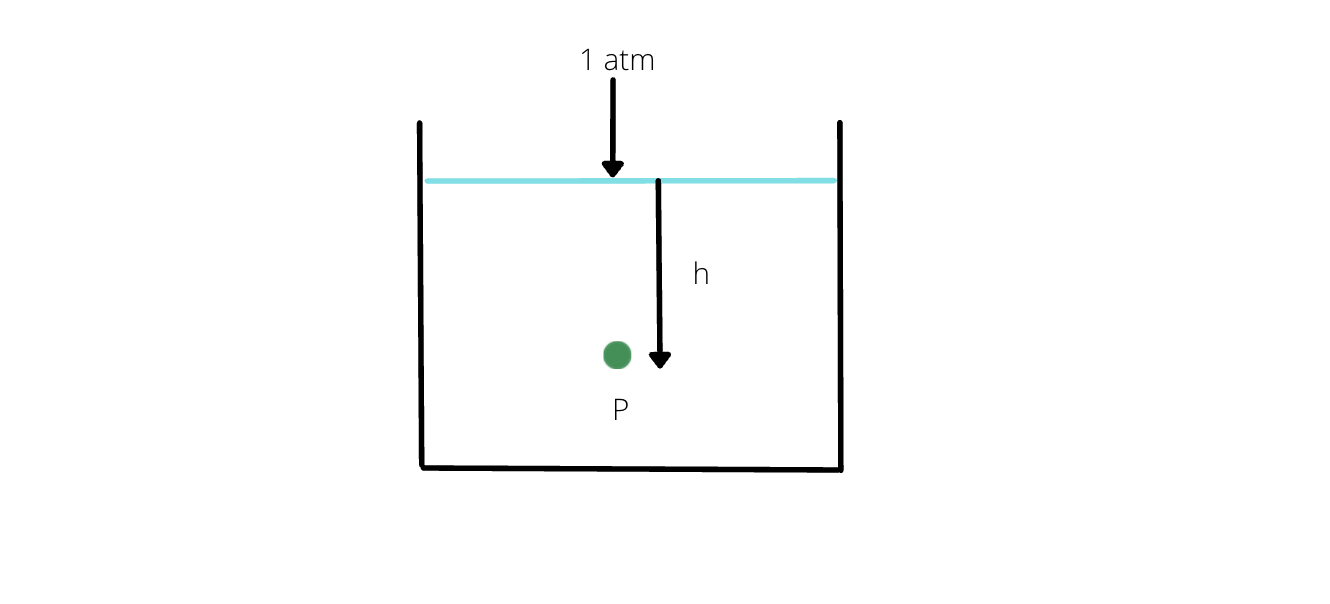
- Pressure at a depth 'h' of a liquid:
Let the density of a liquid be ρ, mass be M and volume be V.
Now, we know that pressure can be calculated as:
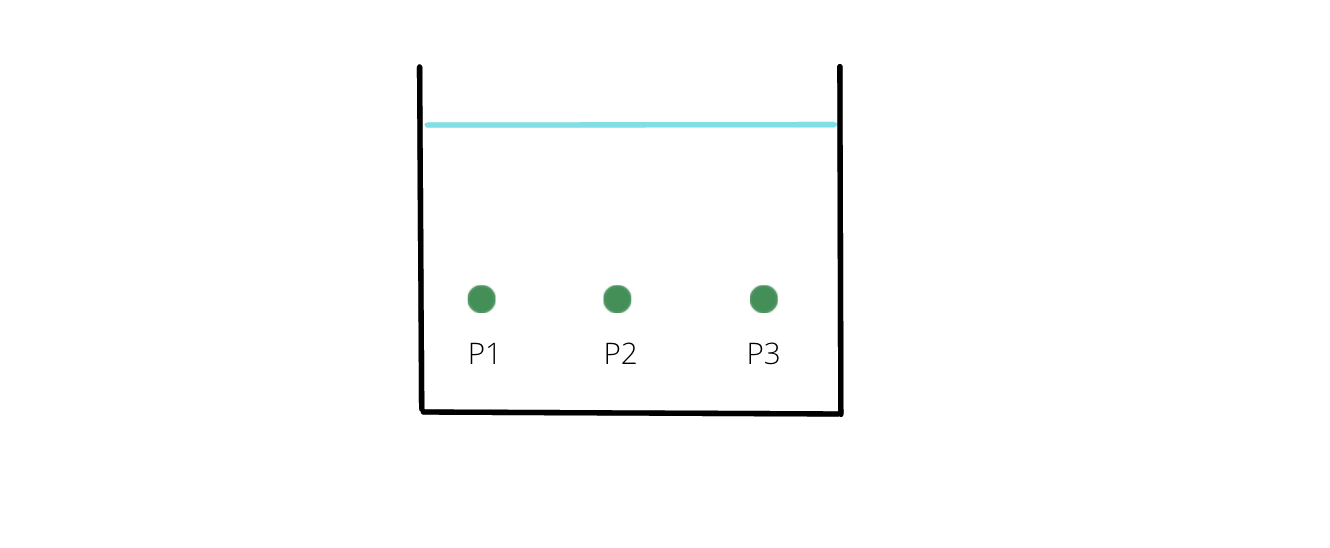
- Pressure is same at the same horizontal level.
- For an open container, at depth 'h' of a fluid, net pressure is:
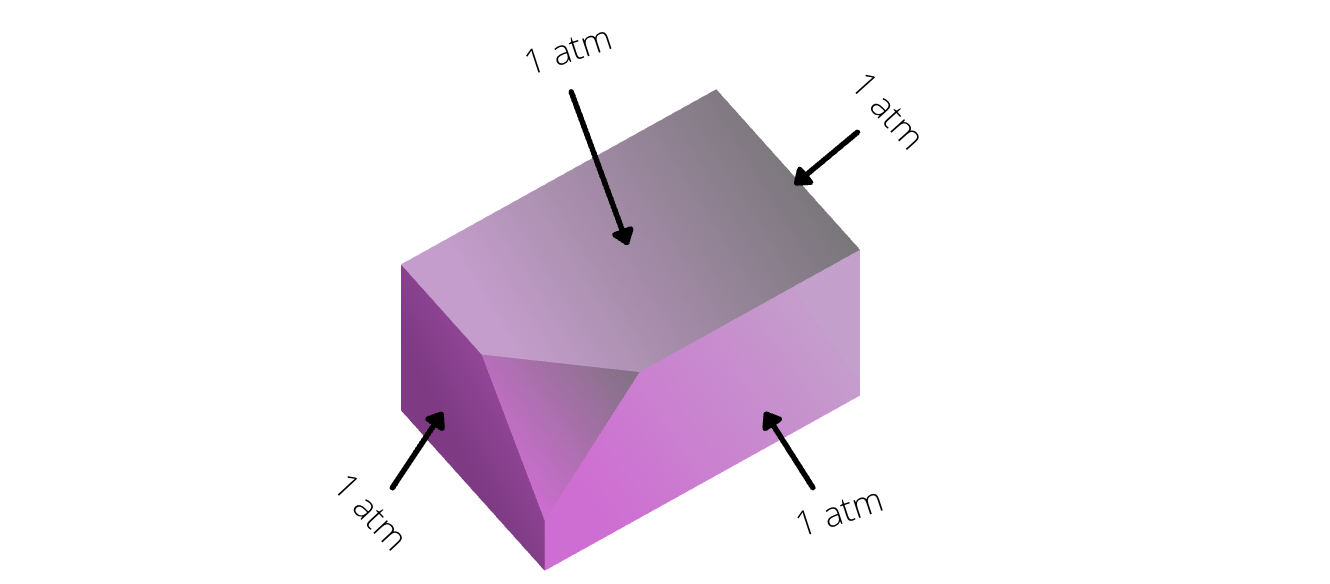
- Pressure is same at all faces of a container. In other words, there exists a same external pressure for a container.
Measurement of Pressure#
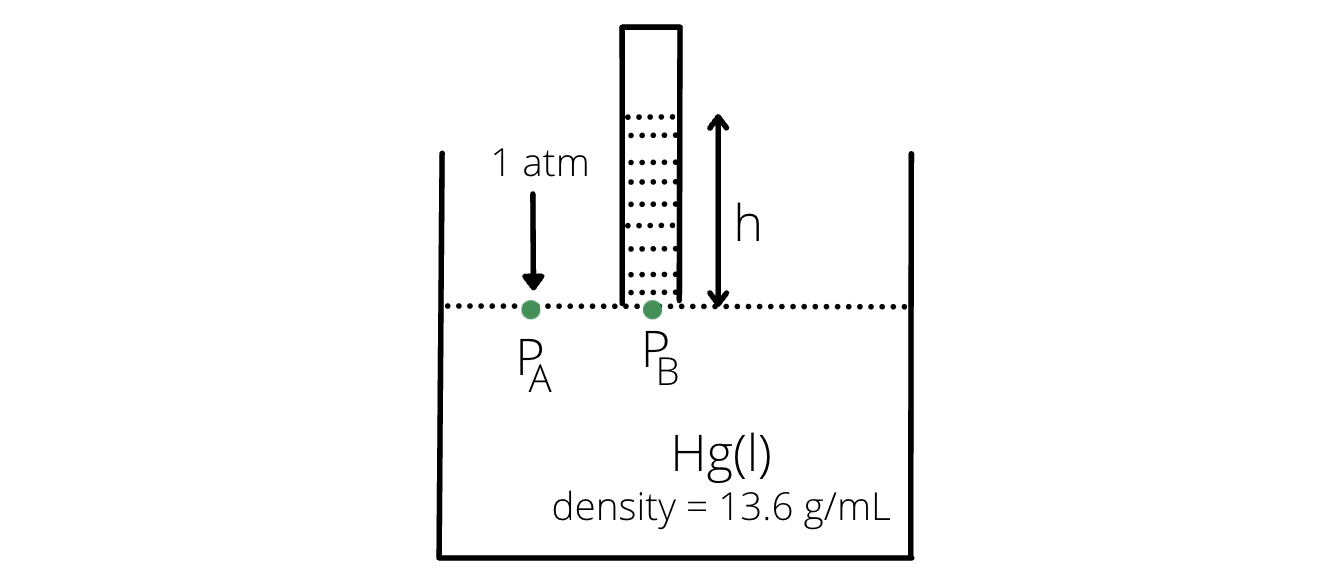
- Let us consider a container filled with liquid mercury at 1 atm external pressure and let a test tube be placed inverted above the surface of the liquid.
Note
Water cannot be used for measuring pressure because the height of water will be very large, so it will be difficult to get such a ling test tube.
If water is used, height of test tube, hw is calculated as: