Periodicity | Metals, Non-Metals and Metalloids#
Periodicity | Metals, Non-Metals and Metalloids#
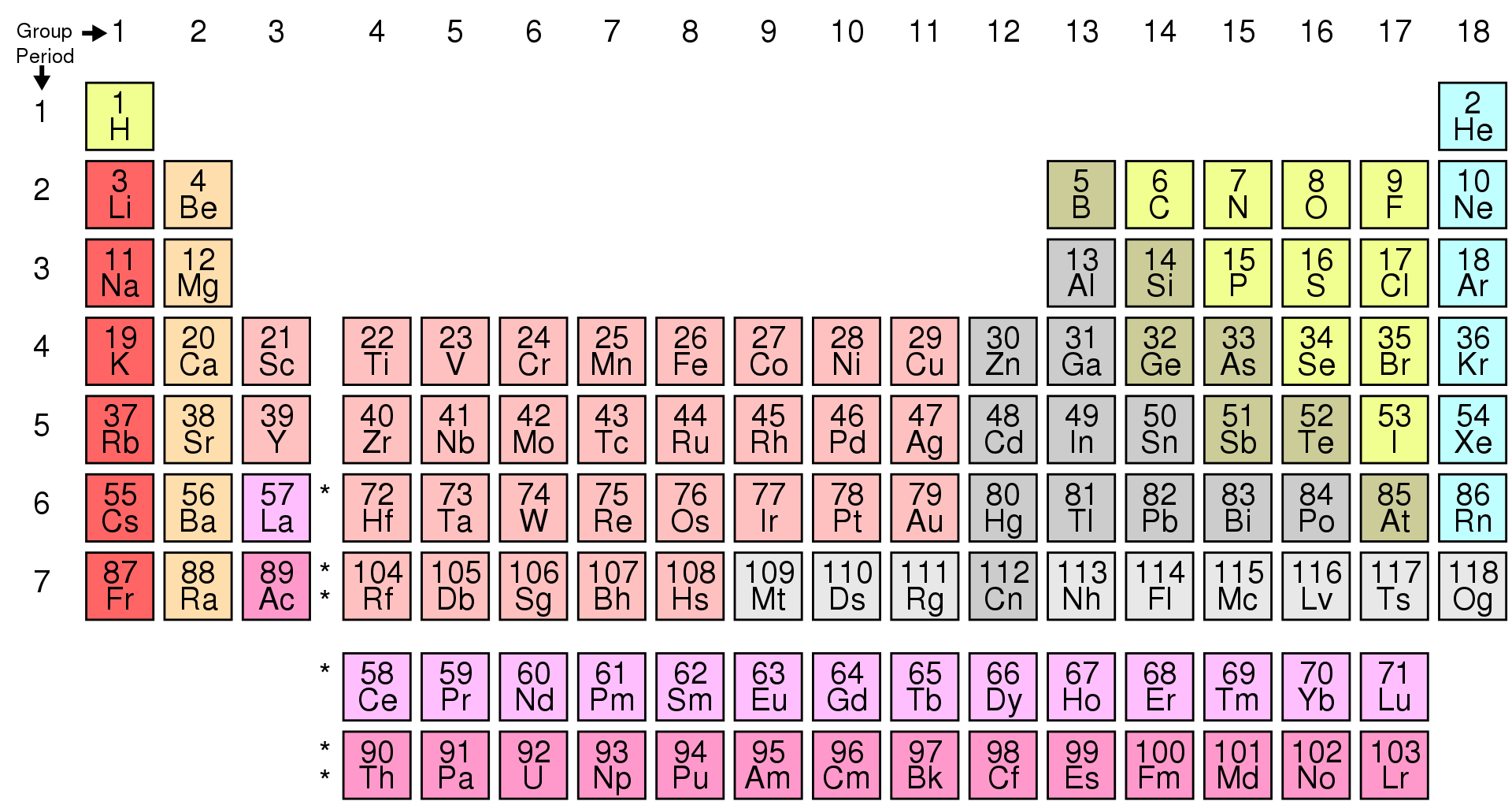
In addition to dividing elements into s, p, d and f blocks, elements can also be divided into three categories: metals, non-metals and metalloids.
Differences between Metals and Non-Metals#
| S.No. | Metals | Non-metals |
|---|---|---|
| 1. | Metals generally lie on left side of periodic table. | Non-metals generally lie on right side of periodic table. |
| 2. | Generally, solid at room temperature except mercury which is liquid. | Generally, solid or gas at room temperature. |
| 3. | Good conductors of heat and electricity. | Bad conductors of heat and electricity. |
| 4. | They have high melting and boiling points except gallium and caesium which have low melting point. | They have low melting and boiling points with the exceptions of boron and carbon. |
| 5. | They are malleable (can be flattened into thin sheets by hammering) and ductile (can be drawn into wires). | They are brittle and are neither malleable nor ductile. |
| 6. | Metallic character increases down the group and decreases across the period. | Non-metallic character decreases down the group and increases across the period. |
| 7. | Examples: Na, Al, Ca, K | Examples: S, O, C, N, He |
Metalloids
The transition from metals to non-metals on moving from left to right across the periodic table is not abrupt. There are some elements whose characteristics lie between metals and non-metals and are called semi-metals or metalloids. The examples of metalloids are silicon, germanium, arsenic, antimony and tellurium.
Image Credit: https://en.wikipedia.org/wiki/Periodic_table
Question#
Considering the atomic number and position in the periodic table, arrange the following elements in the increasing order of metallic character : Si, Be, Mg, Na, P.
We know that metallic character increases down the group and decreases across the period.
So, the order of increasing metallic character is P < Si < Be < Mg < Na.